Surgeons
The Bio-eye hydroxyapatite (HA) orbital implant is a spherical (ball-shaped) implant composed of natural coralline hydroxyapatite. It is used to replace the volume of the orbit when the eye is surgically removed, or as a replacement implant in patients with a poorly functioning, pre-existing implant. For the last 100 years, various materials have been used for these purposes. In recent decades, most orbital implants have been made from silicone or plastic. Historically, the use of nonporous synthetic orbital implants has led to complications such as exposure, extrusion, migration, infection, poor motility, and poor cosmesis.
Physical Comparison of Bone and HA:
-
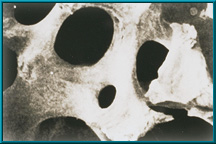
- Scanning electron microscope image (SEM) of human cancellous bone
-
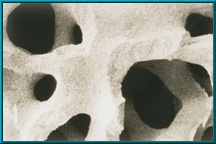
- Scanning electron microscope image (SEM) of coralline hydroxyapatite
Since 1985, natural porous hydroxyapatite (abbreviated HA) has been used as an orbital implant material because it has unique properties. Hydroxyapatite derived from ocean coral is uniquely suited to implantation since both its chemical and physical structures closely resemble those of human cancellous bone (see above). Other implants do not.
Hydroxyapatite was first used as an orbital implant in 1983. In August 1989, the FDA cleared the first HA orbital implant, now known as the Bio-eye Hydroxyapatite Orbital Implant, or simply “The Bio-eye Orbital Implant”. These spheres are completely porous throughout their structure and have an average pore size of 0.5 mm. Natural porous hydroxyapatite is very much like human cancellous bone. The advantages of using natural porous hydroxyapatite as an orbital implant include:
- Decreased lower-lid sag due to the peg’s support of the weight of the artificial eye
- Potential for direct coupling with the artificial eye to make the artificial eye move in conjunction with the normal eye
- Decreased migration
- Decreased extrusion
- Resistance to infection
The Bio-eye Hydroxyapatite Orbital Implant has gained wide acceptance among ophthalmologists and oculoplastic surgeons around the world as the implant of choice for enucleation, evisceration, and secondary implantation (Hornblass 1992). The two most comprehensive studies to date reported very low rates of complications related to exposure in patients where the natural hydroxyapatite orbital implant was used. Shield’s (Shields 1993a) study of 200 cases revealed only 3 (1.5%) exposures, which were easily managed, and no cases of infection. Hornblass’s (Hornblass 1992) comprehensive survey of ophthalmic plastic surgeons did not specifically cite a rate of exposure but did report rates of associated extrusions and infections of less than 2%. Traditional synthetic orbital implants, by contrast, were reported to have infection rates of 7 to 10%, and extrusion rates of 9 to 42%. (Shields 1993a)
The Bio-eye Hydroxyapatite Orbital Implant can be used in primary enucleations, eviscerations, and as secondary implants (in patients who have had their eye removed many years ago).

